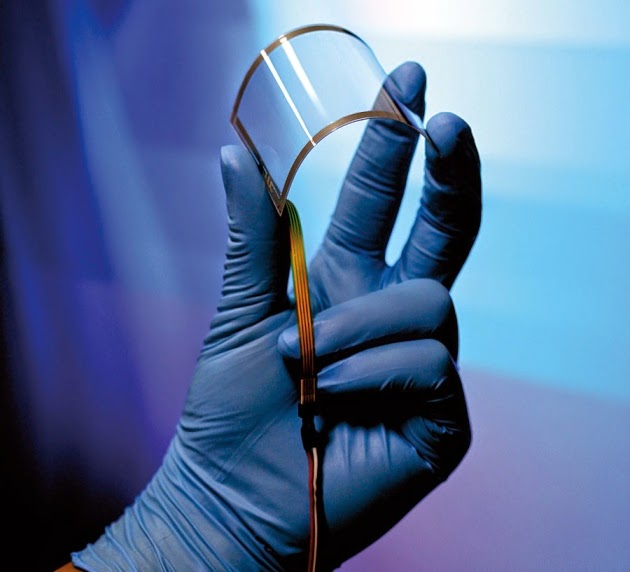
Scientists have
created the thinnest, lightest solar power cells yet — so lightweight that they
can be draped on top of a soap bubble without popping it.
The
researchers suggested that these ultrathin solar cells could be placed on almost any solid surface, including fabric,
paper and glass.
Solar
cells, technically known as photovoltaic cells, directly convert energy from
light into electricity.
The new solar cells are as small as 1.3 microns thick. In comparison, the
average human hair is about 100 microns thick.
The
new devices are also superlightweight, weighing only about 0.01 lbs. per square
yard (3.6 grams per square meter). In comparison, typical piece of office paper
weighs about 20 times more.
The
idea to drape a solar cell on top of a soap bubble came because "we wanted people to see how
thin this solar cell was, but you can't tell the difference between a 10-micron
and a 1-micron film by eye," said study lead author Joel Jean, an
electrical engineer at the Massachusetts Institute of Technology (MIT).
"My lab mate Patrick Brown suggested floating the cell on a bubble to make
the weight difference much more dramatic, so I tried it. My first reaction to
seeing it was probably a lot like yours — 'Cool!'"
The
new solar cells convert light to electricity with about the same efficiency as
conventional, glass-based solar cells, the researchers said. "It's unusual
for flexible cells to perform as well as rigid cells on glass," Jean told
Live Science.
In
addition, the power-to-weight ratio of the new devices is among the highest
ever achieved for solar cells. This is key to applications in which weight is
important, such as on spacecraft or on high-altitude research balloons, the researchers said.
Conventional
silicon-based solar modules produce about 6.8 watts per lb. (15 watts per
kilogram), but these new devices can generate more than 2,720 watts per lb. (6
watts per gram), or about 400 times as much.
"It
could be so light that you don't even know it's there, on your shirt or on your
notebook," study senior author Vladimir Bulović, an electrical engineer at
MIT, said in a statement. "These cells could simply be an add-on to
existing structures."
The
new cells use an organic compound known as DBP as their primary light-absorbing
material. The solar cells are sandwiched between layers of parylene, a
commercially available, flexible, transparent plastic that is widely used to
protect circuit boards and implanted biomedical devices from environmental damage.
The
solar cells and their parylene supports and coatings are fabricated in a vacuum
chamber at room temperature without the use of any solvents, the scientists
said. In contrast, conventional solar-cell manufacturing requires high
temperatures and harsh chemicals.
The
solar cells and the parylene are grown together. The parylene never needs to be
handled, cleaned or removed from the vacuum during fabrication, which minimizes
exposure to dust and other contaminants that could degrade the performance of
the solar cells, according to the researchers.
The
scientists acknowledged that the solar cell they created to sit atop a soap
bubble might be too thin to be practical — an errant breath could blow it away,
they said. "It's, of course, just for show, but we think it makes for a
good show," Jean said.
The
researchers noted they could easily fabricate parylene films up to 80 microns
thick using commercial equipment without losing the other benefits of their
manufacturing technique.
"Using
this approach, you could imagine laminating lightweight or even invisible solar
cells onto windows or other solid surfaces for building- and device-integrated
electronics," Jean said. "A more robust consumer product might use
these cells laminated onto a conventional flexible plastic sheet, which you
could carry around with you for portable power."
The
researchers noted their fabrication technique can use a variety of photovoltaic
materials beyond the ones they have demonstrated so far. "A more efficient
photovoltaic technology could reach even
higher power-to-weight ratios than the 6 watts per gram that we showed in this
first demonstration," Jean said.
The
MIT team's ultrathin solar cells are almost an order of magnitude thinner and lighter than the
previous record holder, said Max Shtein, a materials scientist at the
University of Michigan at Ann Arbor, who was not involved in this work, said in
a statement. As a result, he noted that this research "has tremendous
implications for maximizing power-to-weight [ratios] — important for aerospace
applications, for example — and for the ability to simply laminate photovoltaic
cells onto existing structures."
It's
not yet known when these solar cells might be commercially available, "but
a general rule of thumb is that it takes a decade for a technology to go from
research lab to market," Jean said. Some of the main challenges in scaling
up this approach for commercial use might include developing an integrated
system for high-throughput manufacturing — for example, roll-to-roll processing
— increasing the deposition speed, and identifying applications where an
ultralight and flexible cell would provide some unique value to the user."
Jean,
Bulović and their colleague Annie Wang, also at MIT, detailed their findings in
the April issue of the journal Organic Electronics.
.